Incorporated in 1995, Page Industries Limited is the exclusive licensee of JOCKEY International Inc. for manufacturing, distribution and marketing of the JOCKEY® brand in India, Sri Lanka, Bangladesh, Nepal and the UAE. Page Industries is also the exclusive licensee of Speedo International Ltd. for the manufacturing, marketing and distribution of the Speedo brand in India.
Financial Results:
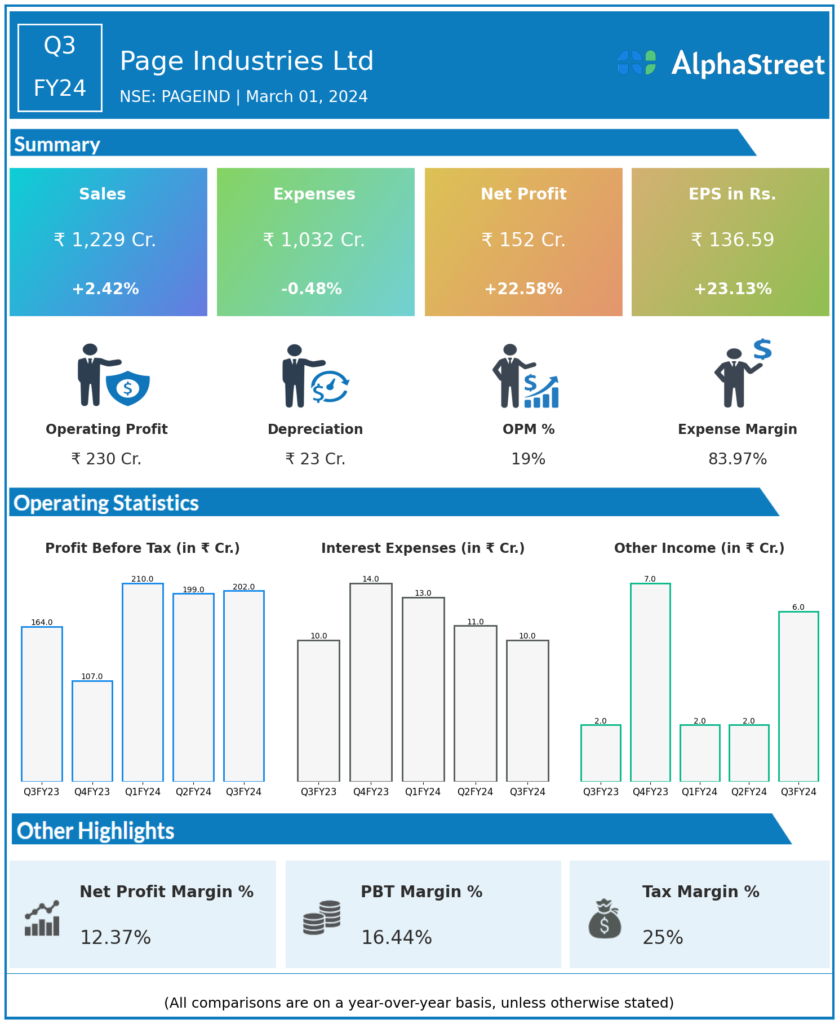
Page Industries Ltd reported Revenues for Q3FY24 of ₹1,229.00 Crores up from ₹1,200.00 Crore year on year, a rise of 2.42%.
Total Expenses for Q3FY24 of ₹1,032.00 Crores down from ₹1,037.00 Crores year on year, a fall of 0.48%.
Consolidated Net Profit of ₹152.00 Crores up 22.58% from ₹124.00 Crores in the same quarter of the previous year.
The Earnings per Share is ₹136.59, up 23.13% from ₹110.93 in the same quarter of the previous year.
*It is important to note that the way the results have been accounted for are slightly different than the ones the companies may choose to publish.
*The presented data is automatically generated. It may occasionally generate incorrect information.
